Multifunctional Porphyrin-based Metal-Organic Frameworks
Flávio Figueira, Filipe A. Almeida Paz
Department of Chemistry & CICECO – Aveiro Institute of Materials, University of Aveiro, 3810-193 Aveiro, Portugal
E-mail: ffigueira@ua.pt
MOFs constitute an outstanding class of crystalline materials [1]. It is easy to imagine a sizable structural diversity amongst these structures, prepared from metal ions or cluster nodes and organic linkers attaining to countless potential combinations between them. The usage of porphyrins (Pors) as linkers in the construction of MOFs has shown great promise in recent years, mainly because of two fundamental driving forces: on the one hand, Pors have intrinsically remarkable properties; on the other hand, their pivotal role in nature in diverse biological functions is also well known [2, 3]. Our research group has been focusing on the development of new MOFs based on linkers bearing phosphonic acid groups coordinated to lanthanide cations [4]. This communication described our most recent efforts to extend our research to porphyrins to prepare functional materials. showing high versatility regarding different applications: photo-degradation of a sulphur mustard simulant, chemosensing of nitro-aromatics and heterogeneous sulfoxidation catalysis (Figure 1) [5].
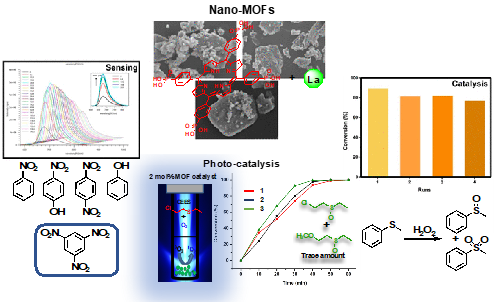
Fig.1. Multifunctional Porphyrin-based Nano-Metal-Organic Frameworks
Acknoledgements: This work was developed within the scope of the project CICECO – Aveiro Institute of Materials, UIDB/50011/2020 & UIDP/50011/2020, financed by national funds through the FCT/MEC and when appropriate co-financed by FEDER under the PT2020 Partnership Agreement. The research contract of FF (REF-168-89-ARH/2018) is funded by national funds (OE), through FCT, in the scope of the framework contract foreseen in Nos. 4, 5 and 6 of article 23 of the Decree-Law 57/2016, of August 29, changed by Law 57/2017, of July 19.
References:
1] P. Silva, S. M. F. Vilela, J. P. C. Tomé, F. A. A. Paz, Chem. Soc. Rev., 44 (2015), 6774.
[2] W. Y. Gao, M. Chrzanowski, S. Q. Ma, Chem. Soc. Rev., 43 (2014), 5841.
[3] S. Shaik, S. Cohen, Y. Wang, H. Chen, D. Kumar, W. Thiel, Chem. Rev., 110 (2010) 949.
[4] A. D. G. Firmino, F. Figueira, J. P. C. Tomé, F. A. A. Paz, J. Rocha, Coord. Chem. Rev., 355 (2018), 133;
[5] C. F. Pereira, F. Figueira; R. F. Mendes, J. Rocha, J. T. Hupp, O. K. Farha, M. M. Q. Simões, J. P. C. Tomé, F. A. A. Paz, Inorg. Chem., 57(2018), 3855.
