Novel metal-phosphonosulphonate materials: Proton conductivity and modelling through MD simulations
Konstantinos Xanthopoulos and Konstantinos D. Demadis
Crystal Engineering, Growth & Design Laboratory, Department of Chemistry, University of Crete, Heraklion, Greece
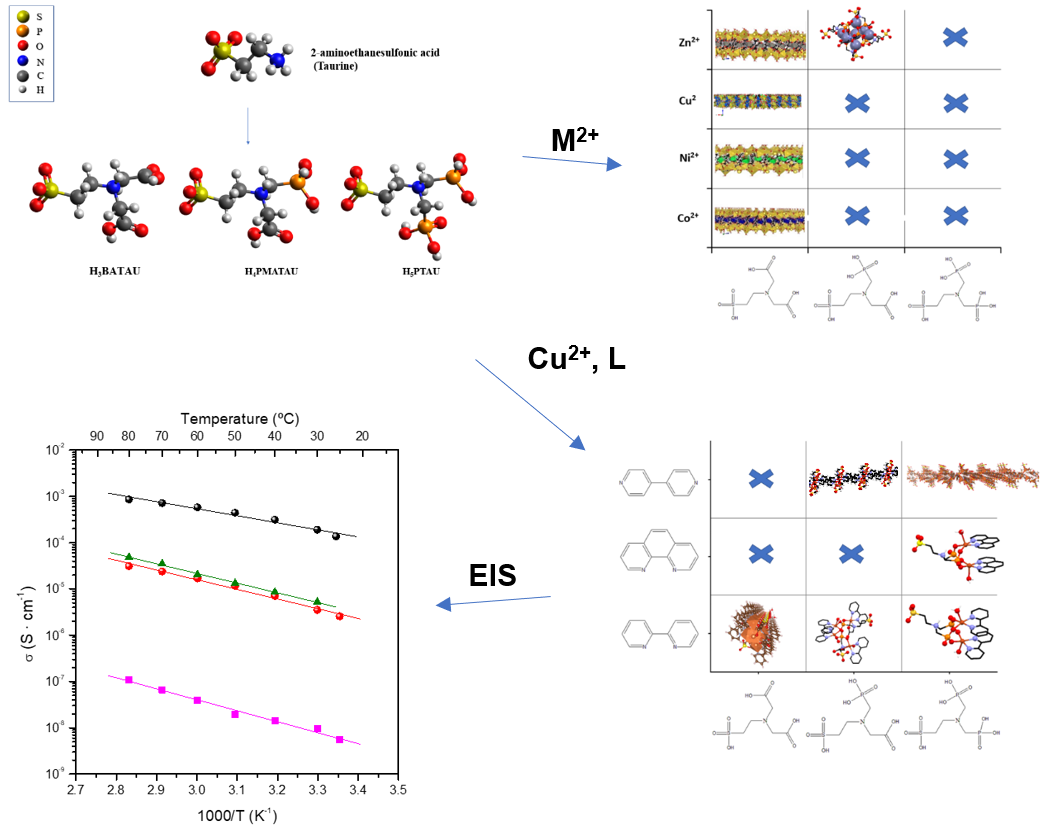
Metal phosphonate chemistry is a growing area in the MOF community due to several attractive features of metal phosphonate materials: (a) thermal and hydrolytic stability, (b) extended structural variability, (c) framework transformations, (d) a multitude of potential applications, etc. One of the recently explored applications is proton conduction, and metal phosphonates are interesting candidates for that, as they provide several acidic sites and well-defined proton conduction pathways. In this work, we report the synthesis of a family of three organic linkers based on 2-aminoethanesulfonic acid (taurine), which contain on their backbone a variable carboxylate/phosphonate ratio. We also report several coordination compounds that are produced with various metal ions, M²⁺ = Co²⁺, Ni²⁺, Cu²⁺, Zn²⁺. These were studied as potential proton conductors using Electrochemical Impedance Spectroscopy (EIS). Furthermore, classical molecular dynamics simulations were used as an additional tool to determine the mechanism of proton conductivity in one of the materials, as a case study.