Unexpected adsorption-induced flexibility in a pillared-layered copper ethylenediphosphonate
Marco Taddei,¹ Roberto Köferstein,² Margherita Cavallo,³ Valentina Crocellà,³ Ferdinando Costantino,⁴ Athanasios Koutsianos⁵
¹University of Pisa; ²Martin Luther University Halle-Wittenberg; ³University of Torino; ⁴University of Perugia; ⁵TU Dortmund
Pillared-layered structures, consisting of dense inorganic layers connected via organic linkers, are ubiquitous in metal phosphonate chemistry. These structures are usually non-porous, because of the high density of organic moieties in the interlayer space that leaves little or no room for small molecules to diffuse and adsorb. In this work, we have thoroughly assessed the CO₂ adsorption behaviour of a recently reported, pillared-layered Cuᴵᴵ ethylenediphosphonate (Cu-EtP)¹ that features narrow channel-like pores (diameter < 5 Å) (Figure 1).
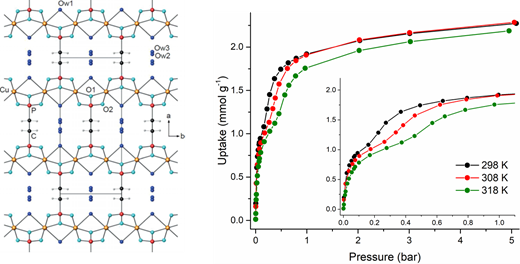
Figure 1. Crystal structure (left) and CO₂ adsorption isotherms (right) of CuEtP.
We found that proper activation conditions are key to fully remove the water molecules strongly bound to Cuᴵᴵ sites. Once properly activated, Cu-EtP has a very high density of open metal sites (0.0093 sites Å⁻³, twice as many as MOF-74) and can adsorb a significant amount of CO₂ at saturation (2.25 mmol g⁻¹, about 5.6 mmol cm⁻³). Most interesting, it displays a step-shaped isotherm, whereby a preliminary adsorption event takes place at pressure below 0.1 bar, followed by a step occurring at higher pressure (Figure 1), during which adsorption kinetics become very slow. Hysteresis in desorption suggests that major structural rearrangements could be responsible for the unusual isotherm shape. The isosteric heat of adsorption extracted using the Clausius Clapeyron equation is in the range of 35-40 kJ mol⁻¹, suggesting a strong physisorptive character. The compound does not adsorb N₂, probably because of its very narrow pores, thus it displays virtually infinite CO₂/N₂ selectivity.
In situ synchrotron powder X-ray diffraction analysis, carried during activation and CO₂ adsorption, proves that the MOF undergoes phase transitions during both stages, with reduction of interlayer spacing upon removal of water and partial reopening of the structure once CO₂ is adsorbed. The phase transitions take place slowly, in agreement with the slow adsorption kinetics observed. In situ IR analysis suggests that CO₂ interacts with the open metal sites generated after removal of strongly adsorbed water and that CO₂ cannot displace water, if this is not fully removed from the surface. NO was also used as a probe molecule, because of its small kinetic diameter and polar nature, to further demonstrate the existence of open metal sites, finding that it is not able to diffuse within the framework when pure, but that it can displace coordinated water.
1 Köferstein, R. et al., Z. Anorg. Allg. Chem. 2016, 642, 1126–1132
