Phosphinate MOF formed from tetratopic ligands as high proton conductivity materials
Matouš Kloda
Metal organic frameworks have been attracting attention in recent years as potential proton conductors. There are two main advantages MOFs have in this application: the possibility to rationally design and tune their properties, and clear conduction pathways resulting from their crystalline structure. We hereby present two new MOF structures, ICR-10 and ICR-11, based on tetratopic phosphinate ligands derived from tetraphenylmethane and tetraphenyladamantane, respectively. The structures of both MOFs were determined by 3D electron diffraction. They both crystallize in the P-3 space group and contain arrays of parallel pores lined with hydrophilic phosphinate groups. The structure of ICR-11 is depicted in Figure 1. The hydrophilic pore walls together with adsorbed water molecules facilitate proton transfer via the Grotthuss mechanism, leading to the proton conductivity up to 4.26∙10⁻⁴ S∙cm⁻¹ for ICR-11.
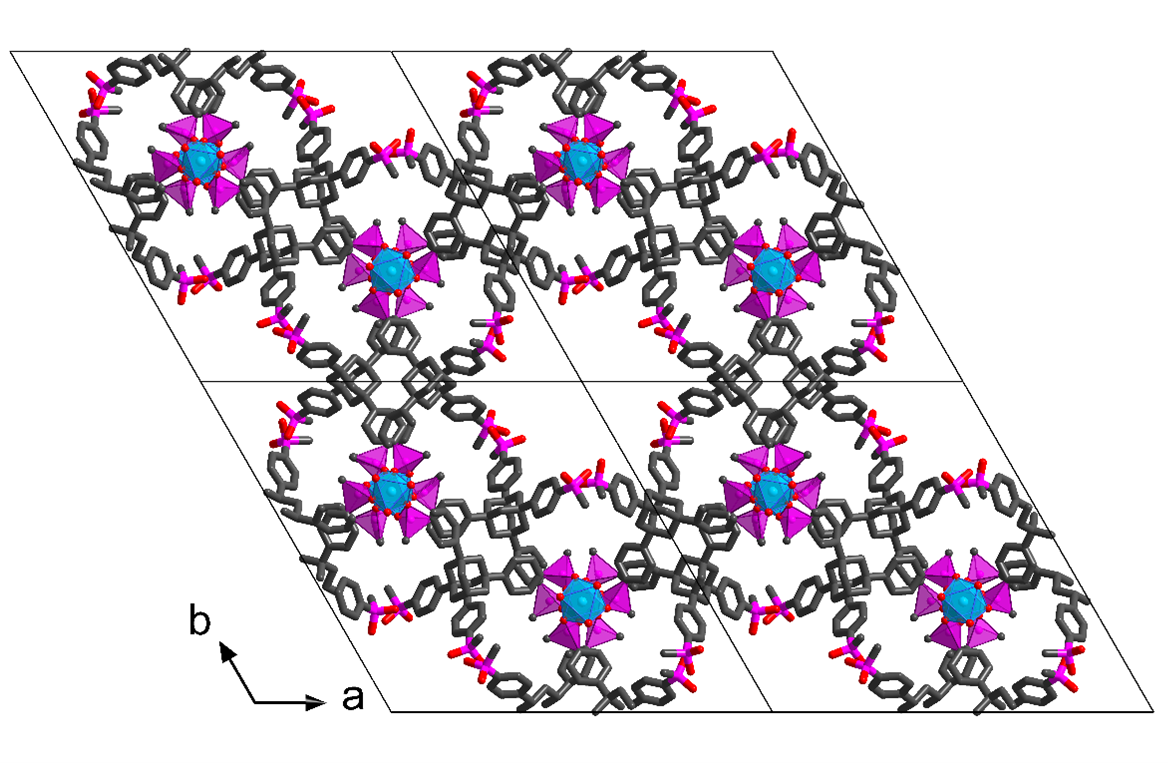
Figure 1: Crystal structure of ICR-11. Hydrogen atoms were omitted for clarity.
