Metal phosphonate materials with aminodiphosphonate linkers of systematically variable molecular size: Synthesis, structures and corrosion inhibition
Argyri Moschona and Konstantinos D. Demadis
Crystal Engineering Growth and Design Laboratory, Department of Chemistry, University of Crete, Heraklion, Greece
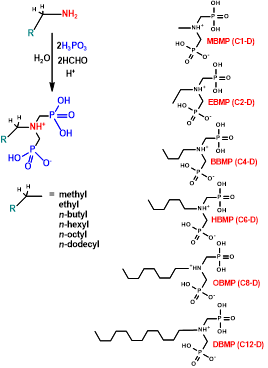
Amino-methylenephosphonic acids constitute an important class of phosphonic acids, which display two phosphonate moieties and a protonated tertiary amine (general formula RN(H⁺)(CH₂PO₃H₂)(CH₂PO₃H⁻)). Because of the stable covalent C-P bond, which protects them from hydrolysis and degradation, they are commonly used as scale and/or corrosion inhibitors in industrial water systems.
In this work, a new family of aminodiphosphonate ligands are synthesized starting from variable-chain length monoamines via the Mannich-type reaction (RN(H)(CH₂PO₃H₂)(CH₂PO₃H), R = methyl (MBMP), ethyl (EBMP), propyl (PBMP), butyl (BBMP), hexyl (HBMP), octyl (OBMP), dodecyl (DABMP)). Alkaline-earth, 3d transition metal and lanthanide ions have been used in combination with these aminodiphosphonate ligands to produce novel 2D and 3D metal phosphonate materials. These have been structurally characterized by single crystal and powder X-ray diffraction.
The efficiency of these metal-diphosphonate systems was evaluated in corrosion inhibition experiments on carbon steel specimens at different concentrations and pH values, based on the NACE Standard TM0169-95 protocol. The metal-phosphonate anti-corrosion coatings exhibit variable performance, depending on the nature of the metal ion and side-chain length of the diphosphonate linker.
Keywords: aminodiphosphonates, metal phosphonates, hybrid materials, corrosion inhibitors