On preparation of phosphinic and phosphonic acids and their derivatives with polyazamacrocycles
Peter Urbanovský, Jan Kotek, Ivana Císařová, Petr Hermann
Department of Inorganic Chemistry, Faculty of Sciences, Charles University, Hlavova 8/2030, 128 43 Prague 2, CZ.
urbanop@natur.cuni.cz
Derivatives of phosphorus acids (i.e., phosphinic and phosphonic acids, PINs and PONs, respectively) are valuable precursors and components widely utilized in various applications such as those in agriculture, hydrometallurgy, surface modifications and functionalization, supramolecular chemistry, metal-organic frameworks, proton conductors, (radio)pharmaceuticals, and medicinal diagnosis.¹ Among all PINs and PONs, those containing α-amino group stand out because of their biological activity at sub-nanomolar concentrations and versatile coordination properties.² Syntheses of PINs and PON have been thoroughly investigated over the last century. Aminophosphinates and aminophosphonates are often prepared from phosphorus precursors (i.e., H₃PO₂ and HOP(OR)₂) via phospha-Mannich reaction in strongly acidic aqueous solution and Kabachnik–Fields reaction under anhydrous conditions, respectively, but the reactions commonly provide hard-to-purify mixtures. The simple user-friendly preparations of aminophospho(i)nates are not readily available.
In this work, amino-H-phosphinic acids (Figure 1) were cleanly formed in “wet” AcOH from commercial aqueous H₃PO₂ in great conversions and isolated yields. The scope of reaction was found to be versatile, and many uncommon structural motives were prepared. Moreover, the reaction outcome strongly depended on nucleophilicity of amines and only those with pKₐ > ~8 provided corresponding AHPAs. Reaction was also investigated by NMR and a tentative mechanism involving N-acetoxy-/N-hydroxymethylamines was proposed.
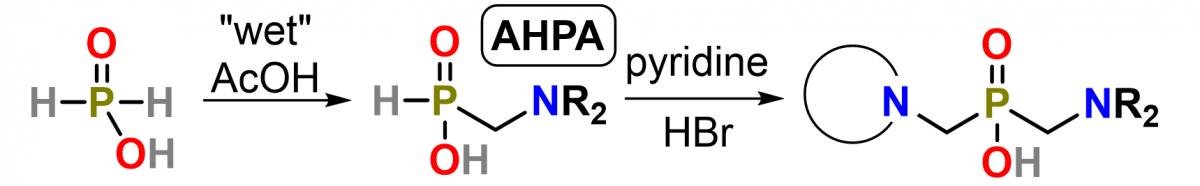
Figure 1 – Scheme of amino-H-phosphinic acid preparation and its attachment to macrocycle amine group.
The prepared AHPAs and HOP(OEt)₂ were further employed in Kabachnik–Fields reaction to synthesize N–C–P–C–N-containing compounds. Among all, pyridine stood out as an optimal solvent for reactions due to efficacy of reaction, excellent solubility of reactants, increased stability of products, and “buffering effect” for the necessary acidic catalyst of reaction. Commercial pyridine hydrobromide was found to be the best source of H⁺ ions. Reaction was further investigated by NMR and the generally accepted reaction mechanism involving formation of aminal and its decomposition to imminium cation was confirmed. Moreover, the optimized conditions were employed to prepare several aminophosphonates and DOTA-based macrocyclic ligands with phosphorus pendant arms (Figure 2) in high yields, exceeding those reported in literature.
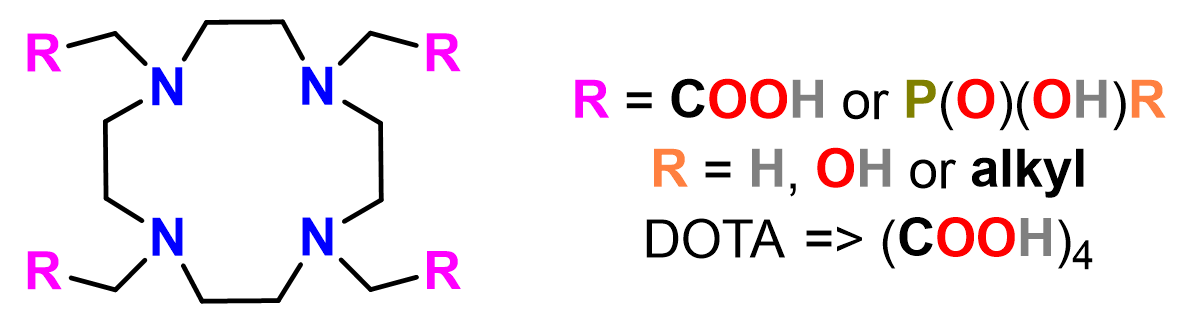
Figure 2 – Structure of polyazamacrocycle with acetate and phosphorus pendant arms.
The synthetized polyazamacrocyclic chelators were further studied as (often novel and pH-sensitive) complexes with Ln³⁺ for radiopharmaceuticals, luminescence, and magnetic resonance angiography.
- Troev K. D., in book: Reactivity of P–H group of phosphorus-based compounds, 1st Ed., Academic Press, 2017.
- Villemin D., Didi M. A. Aminomethylenephosphonic acid syntheses and applications. Orient. J. Chem., 31, 2015, 1–12.
- Urbanovský P., Kotek J., Císařová I., Hermann P. Selective and clean synthesis of amino-H-phosphinic acids from hypophosphorous acid by phospha-Mannich reaction. RSC Adv., 10, 2020, 21329–21349.
