Phosphorus acid pendant arms in polyazamacrocycles: A way how to tune properties of the ligands and complexes.
Petr Hermann
Department of Inorganic Chemistry, Faculty of Science, Universita Karlova, Hlavova 2030, 12843 Prague 2, Czech Republic.
Complexes of polyaza macrocyclic ligands are used in medicine as contrast agents for magnetic resonance imaging (MRI), carriers of metal radioisotopes for PET imaging or for radiotherapy, chelators of fluorescent metal ion for optical imaging, etc. Properties of the complexes (solution structure, magnetic properties, thermodynamic stability, kinetic properties, in-vivo behaviour etc.) as well as metal ion binding selectivity of the chelators are changed through ligand design. These properties are altered by the number of amine groups in the ring, size of the macrocycle and/or kind and a number of coordinating pendant arms. Phosphonic and phosphinic acid groups in the pendant arms are the most similar ones to “standard” acetic acid pendant, and their special properties allow for fine tuning of coordination behavior of the macrocyclic ligands.
Tetrahedral and bulky phosphorus acid groups induce steric hindrances in complexes, e.g. around water-metal ion binding site in complexes causing faster coordinated water exchange or water molecule expulsion; it e.g. leads to increased efficiency of Gd³⁺ complexes as MRI contrast agents or longer excitation lifetime of lanthanide(III) ions. Their high propensity to form hydrogen bonds and ability to serve as bridging ligands in polynuclear complexes alter (de)complexation mechanism(s). The phosphonic/phosphinic acid pendants change acid-base behaviour of the ligands and, thus, thermodynamic stability of the complexes as well as binding selectivity of metal ion in a predictable way. Higher hydration of the groups improves solubility and hydrophilicity of the complexes and, thus, their in-vivo behaviour is changed. High acidity of the groups leads to interaction with metal ions in acidic solutions and it accelerates complexation of metal ions; it is highly desired property for metal radiopharmaceuticals. Bifunctional ligands, suitable for conjugation to biological substrates, can be formed by derivatization of phosphinic acid group far away from metal-binding pocket of the ligands, i.e. without change of coordination properties of the ligands.
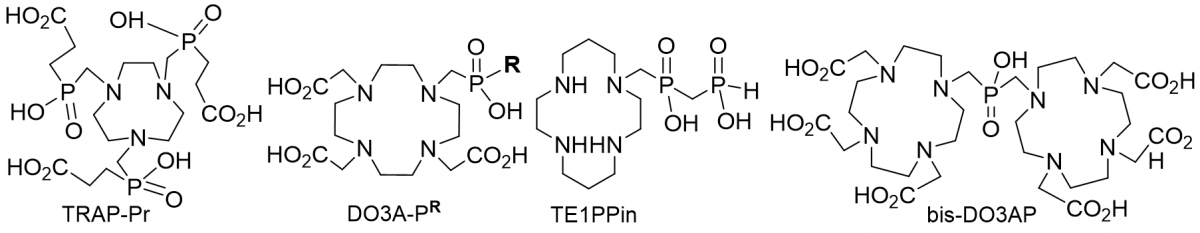
The change of the properties will be discussed for ligands of various macrocycle size and with different pendant arms (examples of the ligands are shown in Figure) and for their complexes. Examples of possible applications will be also given.